The second question arising from this affair is 'who should deal with complications and cockups arising after surgery in the private sector?' This occurs more commonly than most realise. NHS hospitals have greater critical care capabilities and therefore when a complication requiring ITU occurs, the private patient gets transferred to the local NHS hospital. Does the cost of care get claimed from the private health insurance company? I doubt it as insurance schemes only cover a certain length of stay in a private hospital and complications are invariably associated with prolonged lengths of stay.
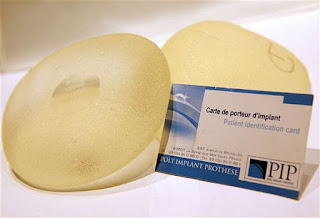
The final and probably most serious question most have asked themselves over the past month is why are regulations for approval of implantable medical devices in Europe so lax ? My speciality of cardiothoracic surgery is very device based - and many others are becoming increasingly so. One of the joys of attending large international Cardiothoracic surgical meetings is the Trade Fair - all those toys for boys that we dream of using but know that we probably won't! The development path of these devices is quite circuitous - Surgeon has an idea, venture capital funded American start up company formed, trials in pigs at local University completed, first human trials in South east Asia, followed soon after by CE marking (identical ironically to Toy approval) and further human trials in Germany (usually Leipzig!). These human trials are then used to generate the data used for FDA approval further down the line. If the device works, approval is granted years after and American patients are then able to receive the device based treatment. The difference between approval systems in Europe and in America is stark. When the device based treatment turns out to be effective, Europeans win out . When the device turns out to be dangerous, American patients win out. Because of the prolonged NICE (National Institute for Health and Clinical Excellence) approval process requiring evidence and NHS cost restraints, British patients tend to fall in between the American and European stools.
Many of the problems associated with European approval are highlighted in a recent radio 4 programme by the excellent Dr. Mark Porter.
No comments:
Post a Comment
I AM SURE YOU HAVE SOMETHING TO SAY ABOUT WHAT YOU HAVE JUST READ, SO PLEASE LEAVE A COMMENT!